Unrestricted for throughput
Few analysis equipments required and suitable for the scenes with different sample sizes.
Product description
This kit is used to detect the presence of SARS-CoV-2 viral RNA in samples collected from Upper respiratory(nasopharyngeal swab, sputum) and Lower respiratory tract(bronchoalveolar lavage fluid). The testing result can be used to aid clinical diagnosis of Covid-19.
Test Method
This kit uses on-step reverse transcriptase-polymerase chain reaction (one-step RT-PCR) by designing primers and Taqman probes(FAM-tag).
Test gene
SARS-COV-2 ORF 1ab, N
Fig. Detection principle
Recommend thermocycler
This kit is suitable for ABI 7900HT, ABI7300, ABI StepOnePlus TM, ABI 7500, ABI 7500 Fast, Stratagene Mx3000P, Roche, Bio-Rad.
Test performance
Limit of detection: 1x103 viral copies/ml
Precision: coefficient of variation(CV)≤10% when performed on the same reference sample for 10 times
Sample storage
This kit needs to be stored at -20±5℃ for no longer than 12 months. For transportation, sealed foam box containing ice packs should be used. Freeze-and-thaw cycles should not exceed 5 times.
CE Declaration of Conformity&CE Certificate
Product Description
After extracting nucleic acid of samples, enrich the probe of reverse transcription, to splice genome sequences of nucleic acid fragments, get the whole genome sequences of novel coronavirus and DNA microorganisms in samples.
Detection contents
Novel coronavirus whole genome sequences
DNA microorganisms
Intended use
1PCR-free (without PCR amplification), No PCR bias, can effectively detect the rare mutation of virus.
2Identify the pathogens of the co-infection in time, to reduce the rate of severe illness and mortality
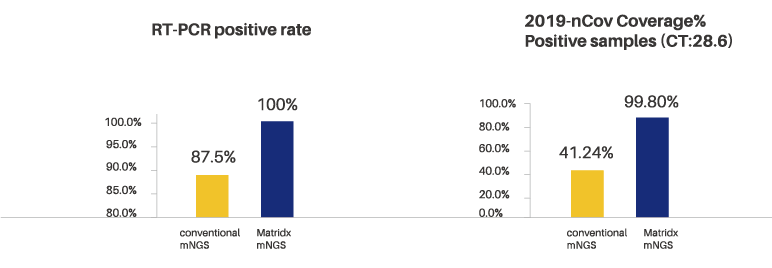
Conventional mNGS VS? Matridx mNGS
For the positive samples of CT 28.6 of qPCR, Matridx mNGS solution has more wide coverage when compare with the conventional mNGS solution (99.8% vs 41.24%), and it’s more easy to detect the mutations.
Process
Purchase information
| Product name | Model/Specification | Item No. |
| Automated Library Preparation Instrument | NGSmaster Pure | MAR002 |
| Microbial homogenizer | BioPrep-6 | AS-13020-00 |
| Microbial genome Data Analysis software | Gente llix | MS001 |
| Single channel automated library preparation instrument | NGSmaster Pure-Lite | MAR002-Lite |
| SARS-CoV-2 Nucleic acid detection | 48 人份 | MD029 |
| kit (Reversible terminator sequencing) | ||
| NGS Library quantification kit | 500 人份/1000 人份 | MD008-P1/P2 |
Constant temperature CRISPR method
Introduction
The product consist of Nucleic Acid amplification analyzer and SARS-CoV-2 Nucleic Acid Detection Kit(Constant temperature CRISPR method)

Application Scenes
Hospitals without PCR lab, Laboratory medicine room (verify the positive samples), Emergency room(in hospital screening), School, Airport, Customs.
Advantages
Operating procedures
Technical Parameter
| Analyzer | |
| Sample size | 0.2ml ? 8wells strip |
| Screen | 7 inch touch screen |
| Interpretation methods | 1. Build amplification curve to get results. 2. The analyzer gets results automatically. |
| temperature uniformity(℃) | Within ±1 |
| Temperature accuracy / control accuracy(℃) | ≤0.5℃ |
| Hot lid function | available |
| Fluorescence intensity detection repeatability | cv≤3% |
| Fluorescence intensity detection precision | cv≤5% |
| Fluorescence linear regression coefficient | ≥0.99 |
| Size(W*D*H) | 145*305*100?mm |
| Net Weight | 2.3kg |
| Reagent | |
| Sensitivity | 100?copies/mL |
| Specificity | no obvious cross-reactivity for common respiratory viruses, respiratory pathogenic bacteria and human swab samples. |
Purchase Information
| Product name | Specifications | Item No. |
| 2019-nCoV Nucleic Acid Detection Kit ( CRISPR-based Nucleic Acid Detection) | 50tests/box | MD027-P1 |
| Nucleic Acid amplification analyzer | FMS-800M | MAR007 |